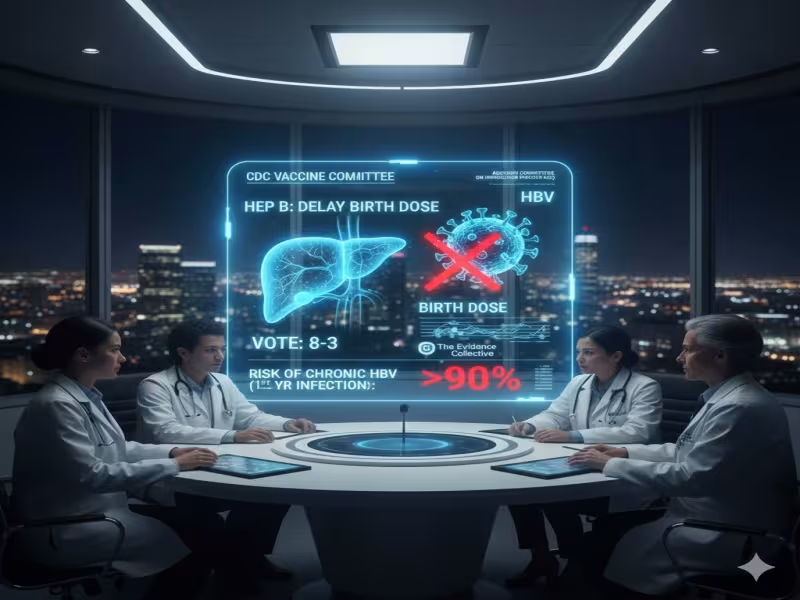
SHERIDAN, WYOMING - December 8, 2025 - The CDC's Advisory Committee on Immunization Practices (ACIP) has triggered a major shift in U.S. newborn immunization policy, voting 8-3 to delay the hepatitis B vaccine for infants born to hepatitis B-negative mothers from the traditional birth dose to two months of age, despite decades of evidence and CDC messaging that the shot is safe, effective and critical for long-term protection.
A Narrow Vote to Delay the Birth Dose for Some Newborns
Under the new recommendation, only babies born to mothers who test positive for hepatitis B-or whose status is unknown-would still receive the birth dose. For infants of mothers who test negative, the vaccine can be delayed until two months, effectively dismantling the universal birth-dose strategy that has been in place for about 30 years.
The decision followed a contentious process. ACIP members were presented with multiple versions of the voting language in the days leading up to the meeting, and even delayed the vote once due to confusion over the wording. Nonetheless, the committee ultimately endorsed the delay for a subset of newborns, even as the CDC's own public information continues to describe the shot as "safe and effective" and to emphasize that most adverse events are limited to local injection-site reactions.
Evidence Still Favors Universal Birth Dosing
The shift comes in direct tension with expert assessments shared ahead of the meeting. In a briefing, The Evidence Collective noted that given the established safety of the hepatitis B vaccine for newborns and the very high risk-up to 90%-of chronic hepatitis B when infants are infected in the first year of life, it remains "entirely reasonable to recommend the HepB birth doses universally." The group also highlighted that before universal screening and birth-dose recommendations, 7-11% of children born to hepatitis B-negative mothers still acquired the virus via household or community exposure.
The CDC itself underscores that "scientific evidence overwhelmingly supports the safety of hepatitis B vaccines," with serious adverse events remaining rare. From a population-health perspective, the universal birth dose has served as a safety net, protecting against missed maternal screening, documentation gaps and community transmission pathways that are hard to predict at the individual level.
Contentious Expert Testimony and a Politicized Panel
The latest decision did not occur in a vacuum. ACIP was reconstituted over the summer after Health Secretary Robert F. Kennedy Jr. dismissed the previous highly vetted panel and installed new members, some of whom have been publicly critical of vaccines. The panel's September meeting already drew criticism for chaotic proceedings and surprise votes that made established vaccines less accessible.
At the December session, presentations again reflected that tension. Atmospheric scientist Cynthia Nevison argued there is "very little evidence that horizontal transmission has ever been a significant threat to the average American child" and described hepatitis B risk as "overstated." Her framing downplayed both the documented burden of early-life infection and the role of a universal birth dose in closing real-world gaps in screening and follow-up. Against this backdrop, committee member Joseph Hibbeln publicly protested the shifting voting language, warning colleagues they were "trying to evaluate a moving target" as they deliberated.
Operational and Clinical Risks for Providers
For hospitals, pediatric practices and health systems, the new recommendation raises practical and clinical concerns. Tying the birth dose to maternal test status increases dependence on flawless documentation and communication between prenatal care, labor and delivery, and newborn services. Any breakdown-missing test results, misclassification, or data entry errors-could leave infants unprotected at a moment when their risk of developing chronic infection is highest if exposed.
In outpatient pediatrics, delaying the first dose to two months introduces new opportunities for missed visits, delayed well-child care, or parental hesitancy to compound risk. Practices that continue to view newborn hepatitis B vaccination as a critical standard of care will need to reconcile ACIP's updated guidance with their own protocols, payer policies and the expectations of professional societies that have long endorsed universal birth dosing.
How Health Systems May Respond in the Near Term
In the short term, the decision will likely produce a patchwork of responses. Some institutions may adhere strictly to the new ACIP wording; others may maintain the universal birth dose as a local standard, citing CDC safety data and long-standing evidence on risk reduction. All will need to reinforce parental communication, clearly explain the rationale for their approach, and document shared decision-making where recommendations diverge.
For payers, public-health agencies and advocacy groups, the vote is a signal that vaccine policy is entering a more politicized era, with expert panels less insulated from controversy and misinformation. For front-line providers, the imperative remains unchanged: protect infants from a preventable chronic infection using tools whose safety and efficacy are well established, even as national guidance becomes more complex.
For official CDC information and updated guidance on hepatitis B vaccination, visit the CDC website.