Dentsply Sirona and High Point University Forge Strategic Alliance to Transform Dental Education
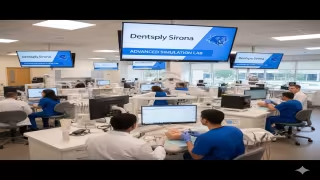
SHERIDAN, WYOMING - June 18, 2025 - Dentsply Sirona and High Point University (HPU) have announced a long-term collaboration that marks a significant step forward in the evolution of dental education, pairing cutting-edge technology with an innovative academic model designed to prepare practice-ready dental professionals.
Equipping the Next Generation with Practice-Ready Skills
The partnership, launching in summer 2025, will see Dentsply Sirona provide advanced equipment and digital solutions to HPU's Workman School of Dental Medicine. This includes fully-integrated Intego Treatment Centers, Primescan intraoral scanners, Primemill units, Primeprint 3D printing solutions, and CEREC software-technologies already shaping modern clinical environments.
Through this initiative, students will gain hands-on training in real-world settings, benefiting from:








