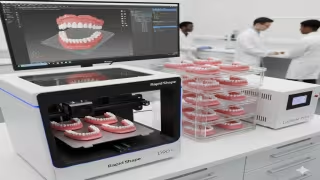
Prototyping and On-Demand Manufacturing Gain Strategic Importance in Next-Generation Medical Device Development
SHERIDAN, WYOMING - November 14, 2025 - Rapid prototyping and digital on-demand manufacturing are becoming indispensable pillars in the medical device industry as manufacturers race to accelerate development cycles, reduce costs, and bring high-performance devices to market faster. With rising demand for personalized medical solutions and increasingly complex regulatory expectations, companies are turning to advanced prototyping partners to validate designs earlier, improve manufacturability, and support real-world clinical readiness.
Bolded H5 subheadings start below
Innovation Driving Faster Medical Device Development