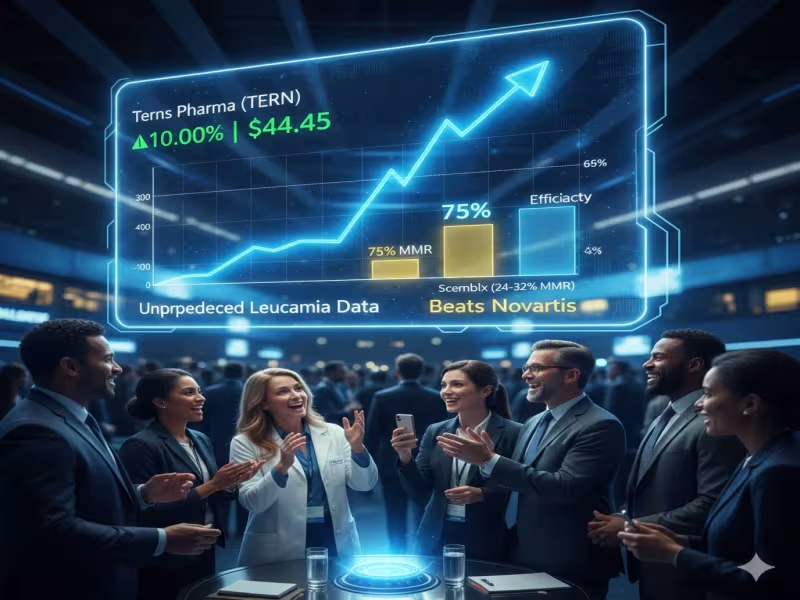
SHERIDAN, WYOMING - December 11, 2025 - Terns Pharmaceuticals is rapidly emerging as a serious contender in chronic myeloid leukemia (CML), after early clinical data for its allosteric BCR/ABL1 inhibitor TERN-701 more than doubled response rates seen with Novartis' approved STAMP inhibitor Scemblix in comparable settings and sent the biotech's share price sharply higher.
Best-in-Disease Early Efficacy Raises the Bar in CML
At the American Society of Hematology (ASH) annual meeting, Terns reported Phase I data from the CARDINAL trial in previously treated CML patients, with 38 participants evaluable for efficacy. TERN-701 achieved a major molecular response (MMR) rate of 75% at week 24, with 64% of patients reaching MMR overall in the dataset presented. The investigational agent also supported simple once-daily dosing without a food effect, improving convenience relative to many existing tyrosine kinase inhibitor (TKI) regimens.
Analysts at William Blair described the result as "unprecedented," highlighting that the compound appears to deliver clear gains in both efficacy and safety, while also offering a more user-friendly dosing profile. The strong readout triggered a sharp move in Terns' stock, which opened up about 10% to $44.45, after pre-market trading had already reflected rising expectations ahead of the ASH presentation.
Outperforming Scemblix and Emerging Rivals on Response Rates
The early CARDINAL data directly sharpen competitive comparisons with both approved and pipeline CML therapies. William Blair's analysis points out that response rates for Scemblix and Enliven's investigational ELVN-001 have fallen in the 24%-32% range in their respective studies, suggesting that TERN-701's 75% MMR at week 24 may represent a step-change in performance.
Importantly, TERN-701 also showed salvage potential for patients who had already been treated with Scemblix and relapsed. In a subset of 10 such patients, 43% went on to achieve MMR on Terns' drug, reinforcing its potential role across multiple treatment lines and resistance scenarios. For clinicians and payers, a single agent that can both outperform existing agents and rescue prior failures would be highly attractive in a disease marked by long treatment durations and periodic regimen switching.
Analysts Model Multi-Billion-Dollar Peak Sales Upside
On the commercial side, equity research firms are already revising expectations upward. BMO Capital Markets called the 75% MMR rate "impressive" and highlighted durability of response as critical to long-term value. Based on the CARDINAL data and the typical multi-year treatment cycles in CML, BMO now projects peak sales of around $3.4 billion for TERN-701, contingent on successful pivotal trials and regulatory approvals.
Because patients with CML often cycle through multiple TKIs over years, high initial response rates that can be sustained translate into meaningful revenue visibility. If the strong Phase I molecular responses translate into longer-term clinical outcomes and can be reproduced in larger, randomized settings, TERN-701 could shift treatment algorithms and displace established options in both second-line and, ultimately, front-line use.
Strategic Pivot from Metabolic Disease to Oncology Pays Off
The TERN-701 data also validate Terns' recent strategic pivot away from metabolic diseases and obesity towards oncology. Previously viewed as an up-and-coming player in obesity and metabolic dysfunction-associated steatohepatitis (MASH), the company chose earlier this year to partner out those assets and concentrate capital and management bandwidth on its CML program, citing an oversaturated obesity landscape.
That decision now appears well-timed. With TERN-701 delivering a clear "best-in-disease" signal in early testing and drawing comparisons that favor it over current and emerging rivals, the program could become a cornerstone asset. William Blair has even raised the prospect of a potential bidding war from larger pharma companies seeking to secure a differentiated CML franchise, given the asset's early efficacy and safety profile.
Next Steps: Toward Pivotal Trials and Potential Deal-Making
Operationally, Terns plans to release additional CARDINAL data in 2026 and engage with regulators on designing a registrational program in both front-line and second-line CML. Management has indicated that the ASH abstract and initial data have energized patient communities, with enrollment momentum in the trial improving substantially-an important factor as larger, later-stage studies come into focus.
William Blair notes that MMR has historically translated well from early-stage to pivotal trials in CML, supporting optimism that TERN-701's Phase I profile can be replicated in Phase III. At the same time, Terns has moved quickly to bolster its balance sheet, launching a $400 million public share offering immediately after the data release to support accelerated development and early launch preparations.
Taken together, the combination of "unprecedented" molecular response rates, salvage activity in Scemblix-experienced patients, a convenient once-daily regimen and fresh growth capital substantially strengthens Terns' position as it looks to scale TERN-701 into a global CML franchise and potentially reshape the competitive landscape for BCR/ABL1-targeted therapies.
For more information on Terns Pharmaceuticals' pipeline and corporate strategy, please visit the company's investor relations resources and official communications channels.